2.0 SCOPE OF EVALUATION
The intent
of this study was to evaluate by impartial objective
means-the ability of Biological Control’s Microcon™ to
filter a simulated human geverated bioaerosol intended
to mimic the type of aerosol produced by a human cough
or
sneeze.
The particulate medium chosen to stimulate
the bioaeorsol was Arizona Road Dust. The test
dust had a mean diameter of 0.76 microcons. This medium
was chosen
because it’s aerodynamic
properties are within the human respirable range. This
medium also permits sufficient residency for consistent and reproducible
measurement of airborne concentrations.
In general, the
test consisted of generating an airaborne dust concentration
in the test room and measuring the change in the perticulate concentration.
The internal volume of the test isolation room and regualr patient room were
1,090 ft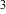 and 1,612 ft and 1,612 ft , respectively. , respectively.
Prior to the start of each test the particulate monitoring equipment was
adjusted and a consistent airborne dust concentration generated. After an
appropriate period of time to allow for stablilization of dust concentration
the Microcon™ was placed in as central location as possible, within
the test room, prior to the start of the test.
Once a stable airborne dust
concentration was achieved and a baseline established the Microcon™ was
then activated. The concentration of airborne dust within
the test rooms was monitored using four (4) direct-reading portable aerosol
monitors which measured airborne particulate levels at two levels in each
of two locations. The monitoring equipment chosen for monitoring particulate
levels were battery
powered hand-held
Haz-Dust™ Particulate Monitors manufactured by Environment Devices
Corp. Thae Haz-Dust™ monitor has a digital read out as well as a
DC voltage output for data recording.
Four (4) of these monitors were used
in each test. Two (2) test stands were used in each test cell and each
contained two (2) monitors mounted at different heights.
Monitors 1 and 3 were mounted on test stand number 1 with monitors 2
and 4 mounted on
test stand number 2. Monitors 1 and 2 were mounted at a height of 66
inches with monitors 3 and 4 mounted at a height of 32 inches. The height
of 66 inches was chosen as a representative of the breathing
zone height of the average standing adult. The height of
32 inches was chosen as an approximation of the height
of a supine patient’s breathing zone.
The test stands were located as follows:
| 1) Test stand #1- |
(holding monitors 1 and 3) was placed
near the front side of the bed approximately 36 inches
from the wall near the head of the bed. |
| 2) Test stand # 2 - |
(holding monitors 2 nad 4 ) was placed in the corner,
at the foot of the bed, near the window approximately
24 inches from the corner of the rooms. |
The test protocol was designed to address possible increased
settling attributable to the greater density of the
test dust as compared to
that of a bioaerosol.
Initially, in an effort to compensate for the lack of air currents
necessaary for uniform paticulate dispersion, one (1)
24 inch diameter fan was employed to distribute the test
dust
and to porduce a more uniform concentration of the
test dust within the test room. The primary reasons for
the
diminution of thetest dust concentrations appeared
to be impact
losses on fan blades, associated surfaces, and gravity induced
settling or drop-out.
Another
source of loss was the scavenging effect produced by static
charges on the
room’s
interior surfaces.
As a result of scavenging, drop-out and impact
losses thequantity of test dust required to produce a stable concentration
of 10-20 mg/M in each test room was somewhat greater than
calculations indictated. A hand-held air-powered nebulizer
was used to disperse the test dust in each test cell. Once the
dust loading requirements were met, it took five (5) to
seven minutes to achieve a stable and acceptably uniform
test
dust concentration within the desired range. in each test room was somewhat greater than
calculations indictated. A hand-held air-powered nebulizer
was used to disperse the test dust in each test cell. Once the
dust loading requirements were met, it took five (5) to
seven minutes to achieve a stable and acceptably uniform
test
dust concentration within the desired range. |

