OLMSTED ENVIRONMENTAL
SERVICES, INC.
RR 1 Box 480
Garrison, New York 10524
TEL: (914) 424-4077
FAX: (914) 424-3482
July 12, 1993
RE:  Investigation
of Isolation Room with the MICROCON® HEPA Filtration
System. Investigation
of Isolation Room with the MICROCON® HEPA Filtration
System.
 An investigation was made into the effectiveness
of the MICROCON HEPA Air Filtration System. A description
of the equipment is included in this report. The
survey involved sampling the air for microbiological
viable organisms and total particulate levels.
The samples were taken before running the MICROCON® and while the equipment was running. The results
indicate that the MICROCON® HEPA Air Filtration
System was effective at removing airborne particulate
material and viable microbological organisms. These
results appear to support the manufacturers claim
that the filtration system will remove airborne
droplet nuclei produced by persons actively infected
with tuberculosis. An investigation was made into the effectiveness
of the MICROCON HEPA Air Filtration System. A description
of the equipment is included in this report. The
survey involved sampling the air for microbiological
viable organisms and total particulate levels.
The samples were taken before running the MICROCON® and while the equipment was running. The results
indicate that the MICROCON® HEPA Air Filtration
System was effective at removing airborne particulate
material and viable microbological organisms. These
results appear to support the manufacturers claim
that the filtration system will remove airborne
droplet nuclei produced by persons actively infected
with tuberculosis.
Background
 Over
the past decade the incidence of respiratory tuberculosis
(TB) infection has increased steadily in the New
York Metropolitan area. Many hospitals in Newark
have treated patients with actively infectious
TB. Patients with active cases of TB can release
infectious droplet nuclei containing TB into the
air thereby exposing health care personnel in proximal
areas. The risk of infection to the exposed persons
is related to the quantities of TB bacillus in
the air, the duration of exposure, and the general
health of the individual. The Centers for Disease
Control (CDC) has a criteria document that suggests
methods that may reduce the risk of TB exposure.
These methods include; Over
the past decade the incidence of respiratory tuberculosis
(TB) infection has increased steadily in the New
York Metropolitan area. Many hospitals in Newark
have treated patients with actively infectious
TB. Patients with active cases of TB can release
infectious droplet nuclei containing TB into the
air thereby exposing health care personnel in proximal
areas. The risk of infection to the exposed persons
is related to the quantities of TB bacillus in
the air, the duration of exposure, and the general
health of the individual. The Centers for Disease
Control (CDC) has a criteria document that suggests
methods that may reduce the risk of TB exposure.
These methods include;
- Using isolation rooms for infectious persons.
These rooms should be under negative pressure
to the surroundiong areas and should receive
a minimum of 6 air changes per hour
- Providing adequate ventilation to reduec concentrations
of TB in the air.
- Using High Efficiency Particulate in Air (HEPA)
filtration systems for recirculated air.
- Using ultra violet lamps to kill airborne TB
- Provide respiratory protection to expand individuals
in high risk areas such as isolation rooms.
 This
survey involved the evaluation of the MICROCON® Air Filtration System. This system consists of
a fan that pulls air into the top of the equipment
through a HEPA filter and discharges the air back
into the room through the base of the equipment.
The unit separates at the three fan settings. This
survey involved the evaluation of the MICROCON® Air Filtration System. This system consists of
a fan that pulls air into the top of the equipment
through a HEPA filter and discharges the air back
into the room through the base of the equipment.
The unit separates at the three fan settings.
- 725 cubic feet per minute (cfm)
- 675 cfm
- 400 cfm
 For
a patient room that is 12 foot square and with
a 8 foot ceiling this unit would provide over 20
air changes per hour at the lowest setting. For
a patient room that is 12 foot square and with
a 8 foot ceiling this unit would provide over 20
air changes per hour at the lowest setting.
Methods
 Air
sampling was conducted following NIOSH method 500
for total particulate in air. The 5.0 micron poly
vinyl chloride (PVC) filters were preweighed by
the ITT Hartford Environmental Lab in Hartford
CT. This is an AIHA accredited laboratory. The
Lab report from Hartford Lab is attached to this
report. Air
sampling was conducted following NIOSH method 500
for total particulate in air. The 5.0 micron poly
vinyl chloride (PVC) filters were preweighed by
the ITT Hartford Environmental Lab in Hartford
CT. This is an AIHA accredited laboratory. The
Lab report from Hartford Lab is attached to this
report.
 Fungi
samples were collected on 3% malt extract agar
using Anderson N6 single stage impaction sampler
operated at a flow rate of 28.31 pm. Samples were
collected using the N6 impaction sampler. Sample
Petri dishes were prepared and analyzed by P&K
Microbiology Services of Cherry Hill, NJ. Fungi
samples were collected on 3% malt extract agar
using Anderson N6 single stage impaction sampler
operated at a flow rate of 28.31 pm. Samples were
collected using the N6 impaction sampler. Sample
Petri dishes were prepared and analyzed by P&K
Microbiology Services of Cherry Hill, NJ.
Results
 Both
the dust samples and the fungi samples indicate
a reduction in airborne particulate levels. Table
I summarizes the dust level sampling results. Levels
prior to operating the air cleaner were 180 micrograms
per cubic meter of air. After running the air cleaner
levels were below the limit of detention of 10
mcg/m Both
the dust samples and the fungi samples indicate
a reduction in airborne particulate levels. Table
I summarizes the dust level sampling results. Levels
prior to operating the air cleaner were 180 micrograms
per cubic meter of air. After running the air cleaner
levels were below the limit of detention of 10
mcg/m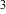 . .
Table
II summarizes the bioaerosol monitoring the fungi
levels in air. Levels were reduced from 40 cfu/m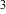 prior
to running the air cleaner to between 5 and 7
cfu/m prior
to running the air cleaner to between 5 and 7
cfu/m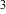 after
running at low speed for approximately 1 hour. after
running at low speed for approximately 1 hour.
Discussion
 The
room tested is designed to operate as an isolation
for Tuberculosis patients. The room is designed
to have 6 air changes per hour and is under negative
pressure to the hallway. This design is adequate
for preventing the speed of airborne TB to the
corridor, but it does not appreciably reduce the
level of airborne contaminants inside the room.
Operating the air cleaner in the room reduced levels
of particulate and bioaerosols. The
room tested is designed to operate as an isolation
for Tuberculosis patients. The room is designed
to have 6 air changes per hour and is under negative
pressure to the hallway. This design is adequate
for preventing the speed of airborne TB to the
corridor, but it does not appreciably reduce the
level of airborne contaminants inside the room.
Operating the air cleaner in the room reduced levels
of particulate and bioaerosols.
The results of this monitoring survey indicate
the ability of HEPA filtration devices to reduce
overall bioaerosol levels inside the isolation
room. These results are consistent with our findings
in a similar test conducted in May 1993. Although
we have not used the system on TB, we believe the
results support the contention that these air filtration
devices are effective at reducing airborne droplet
nuclei.
 There
is no single way to protect persons from TB transmission
and it is recommended thata combination of methods
be used. These sample results indicate that the
HEPA filtration in combination with negative pressure
in isolation rooms will provide an improved level
of protection to the medical staff. There
is no single way to protect persons from TB transmission
and it is recommended thata combination of methods
be used. These sample results indicate that the
HEPA filtration in combination with negative pressure
in isolation rooms will provide an improved level
of protection to the medical staff.
 It
should be noted that the MICROCON® Air Filtration
System is an effective means of lowering the levels
of bioaeosols in the air. There is no engineering
controls that will eliminate TB exposure in the
isolation room. It
should be noted that the MICROCON® Air Filtration
System is an effective means of lowering the levels
of bioaeosols in the air. There is no engineering
controls that will eliminate TB exposure in the
isolation room.
 If
you have any questions or need additional information,
please feel free to call me at your convenience. If
you have any questions or need additional information,
please feel free to call me at your convenience.
Sincerely
Edward Olmsted, CIH, CSP
Airborne Particulate Sampling
Room Tested
|
| Sample No. |
Description |
Results
ug/m3
(1) |
|
| 14024 |
Pretest- in room prior to operating the
MICROCON® Air Filtration System |
180 |
|
| 14023 |
Pretset - in the hallway outside room prior
to operating the MICROCON® Air Filtration System |
20 |
|
| 14021 |
After operating the MICROCON®
Air Filtration
System inside the room |
Below 10 |
|
| 14016 |
Field Blank |
<25 ug per filter |
|
* ug/m3 indicates micrograms
per cubic meter
(2) all sample results are corrected by subtracting the blank quantity. |
| |
Microbial
Evaluation
Room Tested
|
|
|
| Sample No |
Location - Description |
Species
Identified |
Results
CFU/M3 |
|
| 9306281 |
Inside room, pretest; before running
the
MICROCON® air cleaner
141.5 liters |
cladosporium
penicillum
yeasts |
42.4 |
|
| 9306282 |
Outside room, pretest; before running the
MICROCON® air cleaner
141.5 liters |
cladosporium |
14.1 |
|
| 9306283 |
Field Blank |
no growth |
|
|
| 9306284 |
Inside room after turning on
the MICROCON® air cleaner
141.5 liters |
no growth |
<5 |
|
| 9306285 |
Inside room after turning on the Microcon® air cleaner
198.1 liters |
cladosporium |
5 |
|
| 9306286 |
Inside room after turning on the MICROCON® air cleaner
424.5 liters |
cladosporium
sterile fungi |
7.1 |
|
|

